Original route published by Vertex
Process optimization proposal for the original route (A) to (4)
Introduction
A quick optimization could be for the first step a Leuckart-Wallach with N-methyl formamide / formic acid which avoids NaBH4 and furnish an already protected amine. An alternative is N-methylamine in ethanol in presence of triethylorthoformate, followed by formic acid and ammonium formate.
Optionally, to reduce the starting material cost, using the dialdehyde and try to make a selective or total protection with the H-ZSM-5 Zeolite in MeOH or EtOH or IPA if there is an influence on absorption / desorption on the Zeolite, followed by a Leuckart or a modified one with MeNH2/HCOOH. But to be interesting with the TPA, the reactions must be chained by reducing the solvent diversity and choosing the right reagents.
Process optimization proposal
Solid supported version
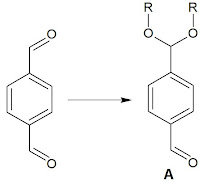
Protection of dialdehyde (A)
Method A
Since this is a symmetric molecule, it is hard to differentiate chemically each carbonyl group. The other way is to try a physical differentiation with Zeolite by the absorption / desorption mechanism during the acetalization.
I have found a publication which use the H-ZSM-5 to make an acetalization of the aldehyde and which show shape selectivity between molecules. Benzaldehyde was used as a model and compared with few others.
Consequently, an acetalization could be tried with R-OH where R=Me, Et, iPr, nPr, nBu (or 2-ethyl-hexanol ?) and so on to check if a mono protection is possible by a physical differentiation absorption between the mono acetal and the dialdehyde. Also, i think the kinetic of the second protection is lower due to the +I effect of diketal to the carbonyl.
Method B
If it is not possible, a total protection could be made by a distillation with a loop “pump-Na¬2SO4 packed column” to remove water from distillate and re-injection of the alcohol to the mixture, or by adding triethylorthoformate to remove water and acting also as a ketalization agent since the zeolite is acidic. In place of zeolite, PTSA/triethylorthoformate/ethanol could be used.
Once the reaction is completed, the catalyst could be filtered (and reused for another batch).
Method C (solid supported synthesis with resin aminomethyl ChemMatrix®)
The setup could be a jacketed column packed with the ChemMatrix® resin swelled with ethanol, pump a solution of TPA dissolved in ethanol and triethylorthoformate stored in a reactor. The circuit should be in a recirculation mode with a inline UV detector to check the TPA concentration.
Reference:
H-ZSM-5-Catalysed Shape-Selective Acetalisation of Aldehydes, M. V. Joshi and C. S. Narasimhan*, Journal Of Catalysis 128, 63-68 (1991).
Advanced Organic Chemistry: Reaction Mechanisms, Reinhard Bruckner, 2002, p290
Reductive amination (2) / protection (chaining possible)
Method A
N-methylformamide method
The simpler way is a Leuckart reaction by adding N-methyl formamide, make a solvent stripping with DMF followed by addition of formic acid, the reaction efficiency must be checked.
Add the ethanolic solution of methylamine and triethylorthoformate to remove water. Once the imine formation is complete, start to make the solvent stripping with DMF or CH3CN (could be made at reduced pressure for acetonitrile, see azeotropic data here), once the solvent stripping is complete, add formic acid and ammonium formate to make the reduction / N-protection. Also it could be tried directly by firstly the ethyl formate distillation and followed by heating to the reflux of ethanol.
The ketal cleavage kinetics with concentrated aqueous formic acid (neat) is relatively slow, also, if ethanol as solvent is used, the probably of ketal removal is probably low since formic acid will be in a stoichiometric amount.
The N-formylation by ammonium formate in refluxing acetonitrile is described. Unfortunately, i didn’t find anything about N-formylation with ammonium formate in ethanol. Lastly, N-formylation could be made in toluene with formic acid and water removal, but i didn’t find anything about toluene and formic acid for the reduction except the enamine reduction of d-camphor with formic acid in 1,2-dichlorobenzene with a good yield, which incite to try with toluene.
Method B1
The challenge is to cleave only one ketal with a catalytic amount of water (since water is regenerated by the imine formation). This is a pure process optimization involving a study (DoE) of the water amount, reaction temperature and solvent which could influence the cleavage kinetic. Optionally, a catalytic amount of PTSA could be used, but seems to be risky.
Method B2
The feasibility of the reaction in these conditions must be checked, and solvent must be identified between DMF, acetonitrile, ethanol or “wet toluene”, most probably at reflux for the two latter. The N-protection could be made by adding the ammonium formate.
The reaction is most probably not 100% selective, but the purification must be made at the next step.
i) In the previous setup, pump the ethanolic solution of methylamine and triethyl orthoformate mixed in the reactor. The circuit should be in a recirculation mode.
ii) Preheat the solution of HCOOH/EtOH in the reactor, preheat the jacketed column too.
Since the imine closer to the resin is... closer to the resin, it is more difficult to make reactions on this part due to steric hindrance, then the reduction occurs preferably on the more accessible site.
A sample loop is needed to take samples for the in process control to monitor the methylamine concentration.
References:
A synthesis of abemaciclib utilizing a Leuckart–Wallach reaction, Michael O. Frederick, Douglas P. Kjell, Tetrahedron Letters, Volume 56, Issue 7, 11 February 2015, Pages 949–951.
Mild water-promoted selective deacetalisatison of acyclic acetals, D. Bradley G. Williams,* Adam Cullen, Alex Fourie, Hendrik Henning, Michelle Lawton, Wayne Mommsen, Portia Nangu, Jonathan Parker and Alicia Renison, GreenChem., 2010, 12, 1919–192.
A convenient method for the N-formylation of secondary amines and anilines using ammonium formate, P. Ganapati Reddy, G. D. Kishore Kumar and S. Baskaran*, Tetrahedron Letters 41 (2000) 9149–9151.
Formic acid reduction of enamines from d-Camphor. A facile route to chiral Bornyl Amines, Rolf Carlson, Asa Nilsson, Acta Chemica Scandinavia, B39, 1985, 181-186.
Activation of 1,3-dioxolane by a protic ionic liquid in aqueous media: a green strategy for the selective hydrolytic cleavage of acetals and ketals, Swapan Majumdar, Mithun Chakraborty, Dilip K. Maiti, Sandip Chowdhury and Jewel Hossaina, RSC Adv., 2014,4, 16497-16502.
A study of the mechanism of certain chemical reactions-I. The mechanism of the Leuckart-Wallach reaction and of the reduction of Schiff bases by formic acid, A. Lukasiewicz, Tetrahedron, 1963, Vol.19, pp1789-1799.
The chemistry of formic acid and it’s simple derivatives, Harry W. Gibson, Chem. Rev., 1969, 69 (5), pp 673–692.
Ketal removal, oximation (3) (chaining possible)
Method A, B1 and B2
The oximation has no needs of water removal on the contrary of imine preparation. Therefore a one-pot ketal cleavage/oximation could be done. Acyclic ketals are cleaved in water at 80°C. With the presence of hydroxylamine sulfate which bring some acidity, a cleavage could be expected.
The protocol could be, on the mixture of the precedent reaction, an addition of a hydoxylamine sulfate aqueous solution followed by heating. The residual formic acid, water and acid salt of hydroxylamine should contribute to the ketal cleavage. If the oximation is slow, some sodium carbonate could be added to liberate the hydroxylamine (pKa = 5,95).
The work-up must be purifying.
Method C
Using the setup of the previous step, in the reactor add hydroxylamine sulfate and water. This solution should cleave the compound from the resin and make the oximation by the same way, heat the solution if necessary.
The cleavage could be monitored by an inline UV detector (concentration of compound = constant)
Once the cleavage / oximation are completed, make in the reactor the Boc protection and isolate the product.
References:
Asymmetric synthesis of N-tert-butoxycarbonyl-amino acids. synthesis of (5S,6R)-4-tert-butoxycarbonyl-5,6-diphenylmorpholin-2-one [(4-morpholinecarboxylic acid, 6-oxo-2,3-diphenyl-, 1,1-dimethylethyl ester, (2S,3R)-)], Organic Syntheses, Vol. 80, 18-30 (2003), Coll. Vol. 11, 399-403.
Mild water-promoted selective deacetalisatison of acyclic acetals, D. Bradley G. Williams,* Adam Cullen, Alex Fourie, Hendrik Henning, Michelle Lawton, Wayne Mommsen, Portia Nangu, Jonathan Parker and Alicia Renison, GreenChem., 2010, 12, 1919–192.
Chlorination of the oxime (4)
(4) Is not isolated and used as is for the cross-coupling step, the solvent could be CH3CN.
Reference:
Route published by Vertex.
Pricing on starting material only
Original route 1400$/kg (199.5$/mol)
Optimized process
N-methylformamide version 303.82$/kg (33.34$/mol)
Methylamine version 586.82$/kg (43.44$/mol)
Disclaimer:
This is some personal works on paper only, i have no responsibility in any way if somebody would try this route and has all sort of troubles, including but not limited to: injuries and money loss. This is for experienced chemists only, and tests must be conducted in a suitable lab only.
But if my work is used to synthesize the targeted molecule described here, please, send a word, even if it fails, chemistry is always an experimental science. This will make me pleased, thank you.
© David Le Borgne, 2015, specialist in chemical process development and optimization.
















No comments:
Post a Comment